In regular cell manufacturing, the quality of raw materials (cells) varies and status of culture changes from moment to moment with conditions and time.
The idea of Quality by Design (QbD) is to analyze such fluctuations, find out the relationship with the quality of the final product, and then set allowable range of fluctuations for successful manufacturing.
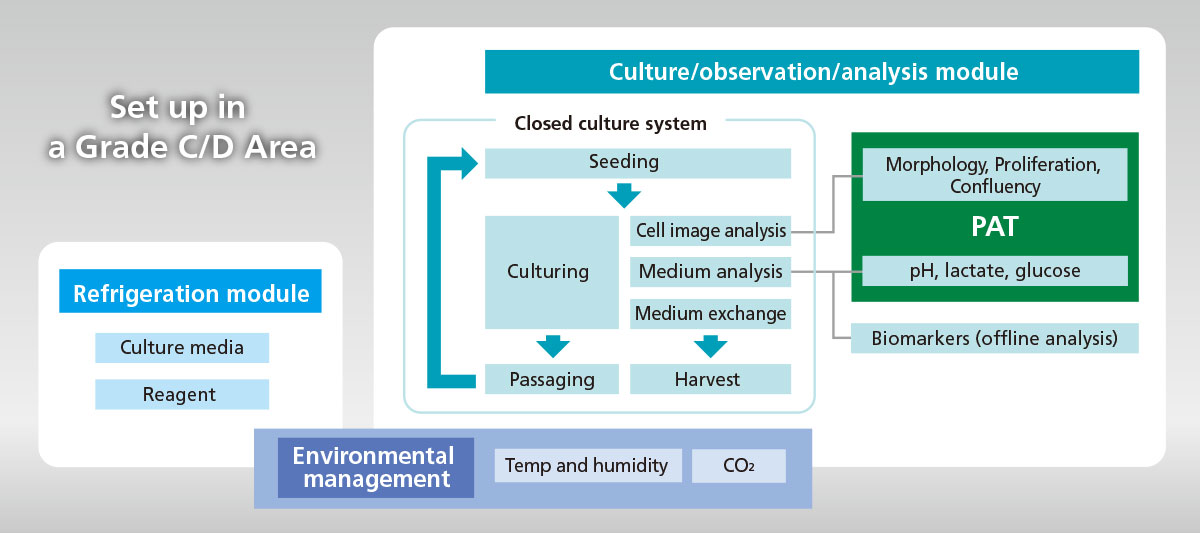
ICP system is equipped with Process Analytical Technology (PAT) to monitor culture status in real time, and stabilizes process by automation; these enable us to manufacture cell products based on QbD concept.
The idea of Quality by Design (QbD) is to analyze such fluctuations, find out the relationship with the quality of the final product, and then set allowable range of fluctuations for successful manufacturing.
ICP system is equipped with Process Analytical Technology (PAT) to monitor culture status in real time, and stabilizes process by automation; these enable us to manufacture cell products based on QbD concept.

※ Joint development with the Foundation for Biomedical Research and Innovation (FBRI) at Kobe
Features
- Fully closed system to secure the asepsis of processes as a whole.
- Designed to use standard multi-layer flasks; scale-up would be feasible with the protocol established with culture dishes or flasks.
- Can monitor cellular image and medium components in real time.
- Auto-sampling port is available for off-line analysis of biomarkers, such as cellular metabolites.
- Can save manpower, eliminate human error, and reduce dependency to skilled staff; all of them will contribute to cost reduction.
- The data obtained by the system which compatible to CSV(Computerized System Validation) will contribute to establish quality management system; it will consistently cover the process from receipt of starting material to product shipping with upper management system.
System Configuration
Specifications
| Cell types | Adherent cells (iPS cells, MSC) |
|---|---|
| Function (culture) |
Seeding, medium exchange, passage, harvest |
| Function (analysis) |
Image observation, culture media analysis |
| Culture container | Multi-layered container |
| Culture surface | Max. 6,300cm2 |
| Shape analysis | CMOS camera |
| Culture media analysis |
Inline: lactic acid, glucose, pH Offline: Biomarkers (2-aminoadipic acid, kynurenine) Measured with another device |
| Power consumption | Typical 2.0kw Max 3.7kw (at 200V) Typical 2.2kw Max 3.8kw (at 220V) |
| Power supply | AC200V~AC240V 1Φ 50Hz/60Hz |
| UPS | Optional (MAX 3,000VA) |
| Ambient environment | Temp: 18-25°C Humidity: 75% or less (no condensation) Cleanliness: Grade C |
| Outer dimensions | Width 2,670× depth 931× height 1,995mm (no protruding parts) |
| Weight | Approx. 1,300kg |
| Air supply | CO2,clean-dry air (both should be 0.3-0.5MPaG) |
Disposable parts
- Disposable closed-system culture kit
- Culture vessels
Movie
We can have a meeting online. You may send your request from the inquiry form.
Please do not hesitate to contact us if you need any further information.


